When you are first diagnosed with ALS, clinical trial participation may not be at the forefront of your mind. Our CEO, Sandra Abrevaya, explains why it should be and why clinical trial conversations are a critical and urgent part of our mission for every ALS patient we serve:
One thing Brian & I didn’t realize when we were first diagnosed six years ago, was that after you develop symptoms, you only have somewhere in the range of 18 to 24 months to get enrolled in a clinical trial. After that, you’re typically excluded from eligibility. That is bringing an additional hardship into a scenario where you are already overwhelmed and facing incredible hardship. So figuring out which clinical trial to get into – as quickly as possible and with the most thoughtful strategy as to which might be most helpful to you—is critically important.
In this overview, we’ll share more about the clinical trial process in ALS, and how we’ve designed Synapticure’s proactive and patient-centric approach to ensure our patients get rapid access to the right clinical trials for them.
In the following we’ll review:
- Why clinical trials are important
- How delays in diagnosis and referral impact trial enrollment
- How date of symptom onset starts the trial qualification clock
- Other common qualification criteria for ALS clinical trials
- Qualifications for trials targeting genetic mutations
- Importance of neurologist involvement in clinical trial discussions
- Goals of different phase trials
- Factors that may influence your choice of a clinical trial
- How our clinical team supports your informed decision-making about trials
Why are Clinical Trials Important?
According to the National Institutes of Health (NIH): “Clinical trials are… at the heart of all medical advances.” Clinical trials look at new ways to prevent, detect, or treat disease; and they assess if a therapy is safe and if it works (efficacy).
To evaluate if a drug works, the US Food & Drug Administration (FDA) assesses if an investigational therapy has a “clinically meaningful benefit.” In essence, can it improve your quality of life, or have a positive effect on how you “feel, function, or survive".
In ALS, clinical trials are especially important because we don’t yet have disease-modifying treatments. Researchers at Massachusetts General’s Sean M. Healey & AMG Center for ALS offer the following perspective on why clinical trial participation matters:
If you or a loved one has ALS, you know the heartbreaking frustration that there are not yet treatments that can stop or significantly slow the disease. You may not know that there are hundreds of scientists and doctors working hard to discover those treatments. That discovery depends, in large part, on the willing spirit of people with ALS who volunteer to be a part of a clinical trial.
Clinical trials help researchers find better treatments for people in the future, but the NIH also acknowledges they “offer hope for many people” fighting diseases like ALS today.
That’s why our team at Synapticure prioritizes discussing clinical trial options with you, helping you optimize your chances of qualifying for the best trial for you.
How Diagnostic and Referral Delays Impact Clinical Trial Enrollment
ALS is a heterogeneous disease in genotype, phenotype and clinical pathology. Getting a timely diagnosis can be challenging. Because of overlapping early symptoms with other diseases, a diagnosis of ALS often requires other diseases that may mimic the presentation to be excluded. That’s why it often takes multiple years and multiple doctors to get a diagnosis. And because most family doctors and local neurologists don’t specialize in ALS, they may not be aware of the exclusionary criteria impacting who can qualify for trials – and why there is a need for urgency.
In a review of 21 studies published from 1990 to 2020, researchers from Cleveland Clinic found the time from symptom onset to diagnosis averaged 10 to 16 months. In a study of 304 people at Massachusetts General Hospital (a leading ALS clinic and research hospital in Boston), the average time—for their patients—was 11.5 months. Diagnostic timelines were shorter for those with fasciculations or slurred speech, and longer for those over 60 years-old and those with sporadic ALS. About half of the patients (52%) were initially misdiagnosed; and each patient saw an average of three different physicians before an ALS diagnosis was confirmed. Led by Dr. Merit Cudkowicz, the researchers cautioned:
“Diagnostic delay prevents people with ALS from entering clinical trials at an early stage in their disease, when they may be more likely to benefit from potential experimental treatments.”
Exemplifying the problem caused by diagnostic delays, a recent review concluded that 60% of people with ALS didn’t qualify for clinical trials. Unfortunately, outside of cities that are research hubs – where there are several ALS Centers of Excellence – the diagnostic delay is often more profound, which in turn, leads to even fewer people who qualify for trials in those communities. Indeed, at a 2021 Congressional hearing, a member of Congress referred to Dr. Richard Bedlack, a neurologist from Duke’s ALS Center of Excellence, who shared that up to 90% of his patients don’t qualify for clinical trials from the first moment they walk into his clinic.
The Date of Symptom Onset starts the Trial Qualification Clock in ALS
Trial qualification dates are calculated from the date of symptom onset—not the date of diagnosis. Most trials limit qualification to those 18 to 24 months from symptom onset, but this time frame can be as early as 12 months. Only one trial has a longer 36-month qualification window: the patient-centric Healey Platform trial.
Notably, when a trial investigator is assessing if you meet the trial criteria, they will look at your past medical records. Your date of symptom onset could be something obvious such as when you began slurring your words, or experienced muscle weakness, muscle twitches (fasciculations), muscle cramping or foot drop. It could be when you started dropping tools at work, couldn’t clip your own nails or had problems gripping a pencil. Or it could also be when you sought medical intervention for a seemingly unrelated procedure like an unsuccessful carpal tunnel surgery, when you received a misdiagnosis of lumbar radiculopathy or when you visited the ER for a broken ankle.
Some people are surprised, then heartbroken, when the trial coordinator shares the determined date of symptom onset that disqualifies people from a chance to access the investigational trial drug. The lesson? We encourage our patients not to delay the trial screening process.
Other Criteria that may Limit your Ability to Qualify for Clinical Trials
Besides the passage of time from symptom onset, other factors like functional capacity, overall health and participation in other trials can influence your ability to qualify for currently-enrolling trials. Exclusion criteria are those characteristics that prevent someone from participating in a trial. Inclusion criteria are those characteristics that a patient must have to participate in a trial. Following are some examples of common inclusion and exclusion criteria:
- Breathing capacity as measured by FVC or SVC (typically 50% - 65%)
- Ability to swallow food, liquid or pills
- Baseline ALSFRS-R above a certain score (typically 30-35)
- Must have both limb and bulbar involvement
- Must have a “definite” or “probable” ALS diagnosis
- No tracheostomy or use of assisted ventilation
- No recent history of certain cancers
- Age limitations (18-60/65)
- No concurrent use of drugs or supplements being tested in other ALS trials
- No exposure to gene or cell therapies used in trials or off-label treatments in the US or foreign countries
- Must be losing an average of 1 point per month on the ALSFRS-R
In this video, ALS clinical trial specialist Dr. Jeremy Shefner of Barrow Neurological Institute’s Gregory W. Fulton ALS & Neuromuscular Clinic outlines the rationale for inclusion and exclusion criteria, as well as the reasons people may want to participate in clinical trials.
Qualifying for Clinical Trials Targeting Genetic Mutations
Another important factor impacting your ability to qualify for clinical trials is whether you are a carrier of genetic mutations known to cause ALS. There are several very promising clinical trials where the mechanism of action targets the damaging effects of those mutations. Thus, genetic testing is critical to determine which trial you might want to explore.
One of our patients shared how Synapticure’s proactive approach impacted her treatment plan and prognosis:
“Thank you for the access to genetic testing! It was discovered that I had a mutation of the SOD1 gene which has completely changed my treatment.”
Notably, some variants are not inherited from either parent so you would not necessarily have a family history. They are called de novo variants. They are common in people with the FUS variants that cause juvenile ALS, a subtype of ALS which impacts people under the age of 25. Without testing, a young person diagnosed with ALS could miss out on a promising Phase 3 trial for Jacifusen currently enrolling people with FUS variants.
Another reason you may not have a family history of ALS is if the gene has incomplete penetrance or variable expressivity:
The same genetic variant found in different individuals can cause a range of diverse phenotypes, from no discernible clinical phenotype to severe disease, even among related individuals. Such variants can be said to display incomplete penetrance, a binary phenomenon where the genotype either causes the expected clinical phenotype or it does not, or they can be said to display variable expressivity, in which the same genotype can cause a wide range of clinical symptoms across a spectrum. Both incomplete penetrance and variable expressivity are thought to be caused by a range of factors, including common variants, variants in regulatory regions, epigenetics, environmental factors, and lifestyle.
For example, ALS-associated genes, C9ORF72 and ATXN2, often have incomplete penetrance, with no symptoms ever showing up in some mutation carriers. So one of your parents may have been a carrier but you would not have known. This could lead to you thinking your ALS is sporadic, and missing out on a clinical trial if you don’t get genetic testing.
For all these reasons, we ensure our patients have access to genetic testing and genetic counseling—regardless if you have a family history of ALS—so you can determine if you would qualify for one of these trials.
With these considerations in mind, Synapticure’s Care Coordinators can start the dialogue about genetic testing and which trials you may want to consider. And if you don’t qualify for clinical trials, our Care Coordinators will work with you to see if there are any options for Expanded Access aka Compassionate Use.
Importance of Neurologist Involvement in Clinical Trial Discussions
Given the rapid pace of innovation in ALS therapy development, many physicians find it hard to stay atop the 100+ ALS trials currently enrolling. Others may only be aware of those trials at their own ALS clinic. Moreover, as this review noted, the biases and preferences of treating physicians can also influence enrollment conversations:
“Earlier studies have highlighted physician preference as the primary reason for non-participation in half of the patients for whom a trial was available and the patient was eligible. Reasons which deterred physicians from recommending trial participation to their patients included limited time and funding resources, treatment preferences and nature of study regimen.”
Just as Dr. Jeremy Shefner, Barrow neurologist and Synapticure’s scientific advisor, emphasized in this video about the clinical trial process: “the most important thing someone can do is talk to their doctor.”
Despite the importance, patients can be left to explore trial options on their own. A recent social media poll asked people with ALS how they first learned about the clinical trial they are participating in. Although it was an informal poll, less than one-quarter of the 327 people learned about clinical trials from a physician, and this further illustrates the need for more neurologist involvement:
- 30% Research on the internet
- 20% Clinicaltrials.gov
- 16% Facebook groups
- 11% Local neurologist
- 10% Neurologist at an ALS Center of Excellence
- 8% ALS non-profits like I AM ALS Signal Dashboard
- 2% Webinars by neurologists like NEALS
- 2% ALS media publications like ALS News Today
- 1% Other people with ALS
- 1% Drug sponsors’ social media
These statistics above are not an anomaly. Rather, they demonstrate why Synapticure’s clinical team prioritizes clinical trial discussions with our patients.
Our neurologists and care coordinators will review your medical history and talk to you about your goals and risk tolerance. After doing a deep dive into the trial data and scientific literature, we will share, in easy-to-understand language, the science and proposed mechanisms of action, and risk-benefit of each trial drug you’re considering. And while we will share our clinical judgment with you, we will not preemptively replace our own opinions for your decision-making.
This vision came directly from our CEO:
When we were first diagnosed with ALS … Brian & I thought:
‘How can we help other families?’ We knew how important it would be to bring together a team of top ALS doctors… to really spend the time you need as a family to unpack the data, to understand all the different trials around the country, not just the trial associated with one clinic… and that has to be explored with people who are willing to go deep on the data, the literature and the science… That’s the kind of team we put together at Synapticure so that every family – not just our family – has access to that kind of cutting edge help!”
Also as Sandra mentioned, because Synapticure has a national footprint – licensed in all 50 states – our neurologists stay abreast of clinical trials across the US. Thus, if geographical limitations aren’t a barrier for you, we will also share trial options with you outside your state.
Our goal is to advise you and provide you with the most information possible so you can make the best decision for you and your family. Then, while you’re participating in the trial, concurrently, our clinical team will continue to be there to support all your care needs.
Understanding Goals of Different Phase Trials
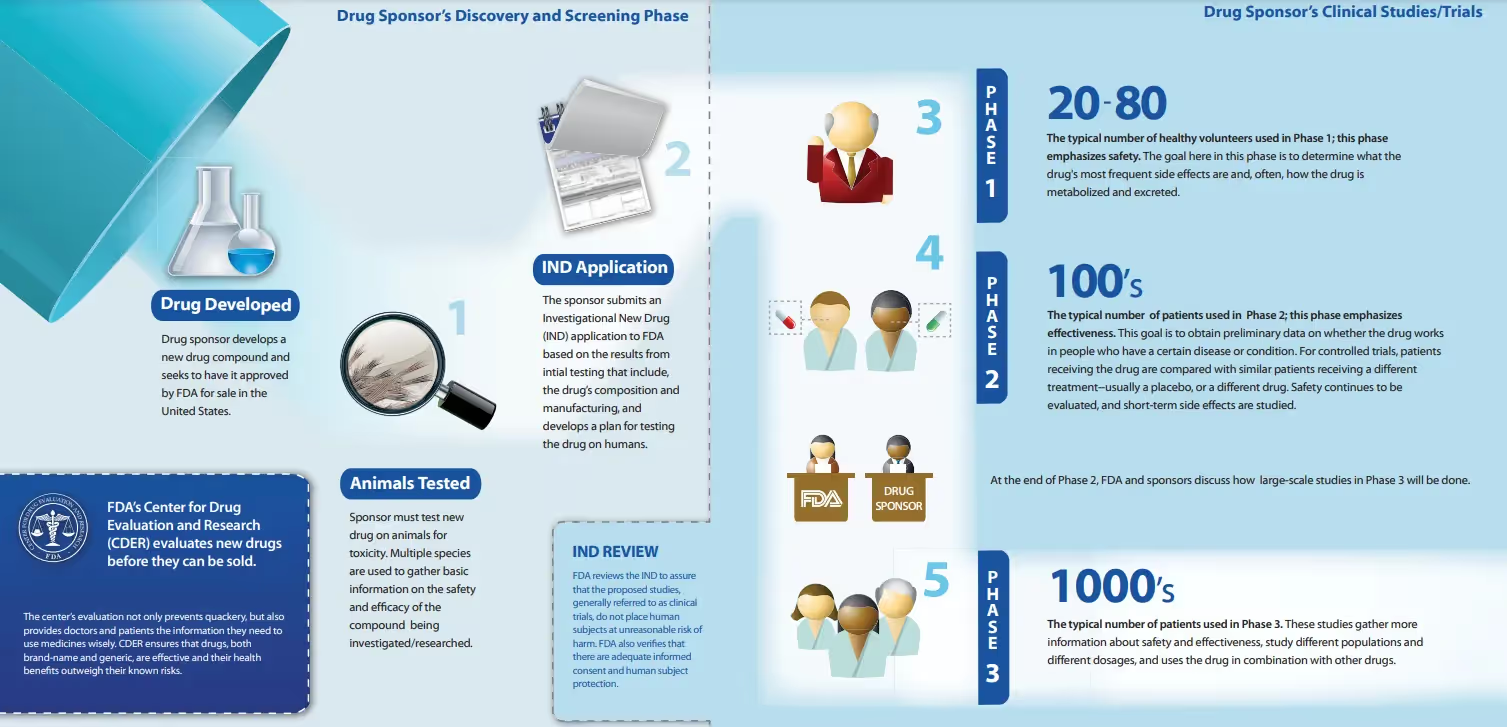
Clinical trials are run in multiple steps, called phases. Each phase has a different purpose and helps researchers answer different questions about an investigational therapy. As a drug progresses through each phase of clinical trials, we learn more about both its safety and efficacy.
To help you understand which trial might be best for you, it’s important to understand the different phases of trials.
- Phase 1 - Phase 1 trials study the investigational drug in humans for the first time. The purpose is to learn about the drug’s safety, to identify side effects, and to determine how the drug is metabolized and excreted. In other diseases, the study population is often 20-100 people. In ALS, the size of Phase 1 trials is typically as few as a couple of people up to 20. Phase 1 trials are not “powered for efficacy,” which means there are not enough people participating in the trial to reach any reliable statistical conclusions about whether it works in humans.
- Phase 2 - This phase emphasizes efficacy. The investigational drug is tested in a larger group of people to determine if there is evidence of efficacy, to assess dosing options, and to further study safety. In other diseases, the study population is typically hundreds of people. In ALS, the size of Phase 2 trials is typically below 100.
- Phase 3 - The investigational drug is studied to determine there is “substantial evidence” to confirm its efficacy, to continue to monitor side effects, to compare it with standard treatments on the market, and to weigh the drug’s risks versus benefits. In other diseases, phase 3 trials enroll thousands. In ALS, the size of phase 3 trials is typically several hundred.
If you Qualify for Several Trials, what Factors should you Consider?
In addition to understanding the science underlying the investigational drug, other factors may influence your decisions. If you qualify for various trials, how do you decide which one is best for you?
First
Many of our patients make that decision based on a trial’s placebo ratio. Placebo controlled trials are considered the “gold standard.” In most Phase 2 and Phase 3 randomized controlled trials (RCTs), there is a control arm, meaning some people get the investigational therapy and some people get a placebo. The chance of getting a placebo varies based on the statistical plan for each trial.
But the decision to forego the risk of a placebo is more complicated than one would think at first glance. For example, would you rather participate in an early Phase 1 trial with no placebo arm, knowing that there is no evidence of safety or efficacy in humans? Such a drug could cause your ALS to progress more quickly or could have lethal consequences. The drug could also cause unanticipated side effects that could impact your quality of life. But the upside is that you could be the first to receive a promising new investigational drug early in your diagnosis when it is most likely to benefit you.
In contrast, would you prefer some data about side effects and some proof that a drug might work in humans, but take the chance of getting a placebo in a Phase 2 or 3 trial? This is a personal decision based on your priorities and risk tolerance.
Second
Another important consideration for many people is whether a trial has an open label extension (OLE), which allows trial participants to continue to get the drug after the randomized, controlled trial (RCT) is over. According to researchers at the University of Michigan Medical School:
Patients who consider enrollment are hopeful that they will derive benefit from the proposed novel therapy…. An OLE reassures participants in a RCT trial that – regardless of which treatment arm they were originally assigned to – they will be provided the study drug without any additional cost … until the trial is terminated or the drug receives regulatory approval.
In ALS, most trials that have OLEs are offered in Phase 3 trials or occasionally in some larger, Phase 2/3 trials. As answered in FAQs by Washington University researchers, OLE is a phase of a study that occurs after the RCT “if a drug is found to have the potential for benefit.” As such, in all but the rarest of exceptions, Phase 1 trials do not typically offer OLE because an investigational drug has no data regarding safety or efficacy in humans.
Some RCTs may also offer a “crossover” trial design, which means you could get the placebo for a certain time period, but then everyone will get the investigational drug for the balance of the trial via OLE. During this time, the trial still continues and the investigators continue to collect data that may be used to support approval. Recently, two drug sponsors used OLE data to support their New Drug Applications for Amylyx's Relyvrio (AMX0035) and Biogen/Ionis’ Qalsody (Tofersen); and the FDA approved both therapies. Thus, OLE can benefit both drug sponsors and patients.
Without an OLE—even if a drug works for you—you will not be able to get the drug until or if it's FDA approved.
Third
A factor many patients consider is the method of delivery of the investigational drug. Is this a pill or liquid you swallow? Can you deliver the therapy via a feeding tube? Is it an IV that you need regularly and if so, can it be done at home? Will you need a port to deliver the therapy or to take repeated blood draws? Is it a nasal spray? Is it an intrathecal injection, and if so, is there any reason it might be more difficult for you to have repeat lumbar punctures? And most importantly, which method of delivery is most likely to ensure the investigational therapy makes it past the blood-brain barrier, which excludes more than 98% of small molecule drugs from getting to the brain?
Fourth
Could the known side effects impact your quality of life? If an investigational therapy causes GI disruptions, that can be difficult for someone who cannot easily move to use the bathroom. Are there any FDA black box warnings of the drug or its ingredients in other diseases? Could a therapy impact your gait at a time when you still have lower limb function? Could it cause pain such as radiculopathy or myelitis that will prevent you from participating in family activities? Is the side effect temporary or does it typically wane with the passage of time?
Fifth
Location is another significant factor impacting which trial you choose. Are you able to travel to the trial site repeatedly? If you have to travel out of town, does the trial compensate you for those costs? If your ALS progresses, will you be able to travel by plane or will you need an accessible van? If you have side effects, would those complications be able to be treated by your local physician or hospital? Could you consider relocating or living with family if a trial site is not close to home? Does the trial gather any data remotely or via telehealth to minimize the burden?
In ALS, the Healey Platform trial has tried to address some of the above issues by ensuring the trial has over 50 trial sites across the US; by minimizing the chance of placebos with a 3:1 drug to placebo ratio; and by offering OLE in its adaptive trial design. We are hopeful that other drug sponsors will adapt their clinical trial designs to patient-centric models.
How Synapticure Helps Patients with Clinical Trials
At Synapticure, our mission is to be patient-centric in all that we do. Because Synapticure was started by an ALS patient and caregiver, our perspective on clinical trials reflects the lived experiences of our co-founders and many on the clinical team who have family with ALS. As Sandra said in the video above, “Your decision should be based on what trial has the best chance—of potentially being useful to you.”
Researchers and regulators caution patients that investigational drugs are not the same as “treatments.” We understand that. But for many of our patients, no risk is worse than ALS. And, trials may offer a possible upside too. If a therapy shows efficacy in a clinical trial, it could provide you access to a “treatment” years before FDA approval. For so many families given a terminal diagnosis, this is hope.
We just witnessed this exact situation with the recent Tofersen trial. As Dr. Timothy Miller presented to the FDA during the Tofersen Advisory Committee meeting, some people in the trial slowed their ALS progression, others regained strength, and many are now outliving the natural history of their disease variant. This is a win in ALS. And we will take every win we can get.
If you are interested in learning more about clinical trial options, we would love to have a discussion with you. You can get started by scheduling a free consultation with one of Synapticure’s Care Coordinators—all from the comfort of your home.
Author: Neuromuscular Specialist Dr. Danielle Geraldi-Samara
Synapticure’s ALS care team is led by Dr. Danielle Geraldi-Samara. For over 15 years, Dr. Geraldi-Samara has focused on diagnosing and treating ALS patients, beginning with her neuromuscular fellowship at the Mount Sinai School of Medicine. Dr. Geraldi-Samara has long-recognized the need for her patients to easily access care at an ALS clinic in their community; and thus, she established an ALS/Neuromuscular clinic from the ground up at NYU-Brooklyn. She also served as a supervising Neuromuscular Specialist at Northwell Health’s multidisciplinary ALS clinic in New York. To read the amazing reviews from Dr. Samara’s patients, click here.











.png)


